Benefit-Risk Balance
We know that "safety" is a relative concept and that decisions about a particular drug's use must be evaluated as a balance between the benefits and the risks of the medication use for an individual patient.
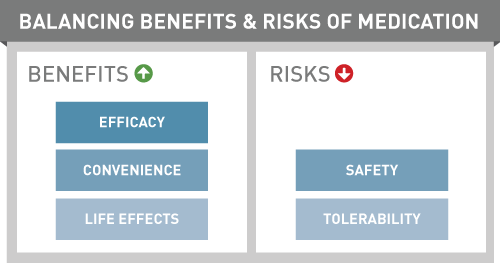
What Is Benefit?
Benefits are positive outcomes or favorable effects associated with a drug. Benefits may be assessed relative to other similar products. Benefits may be due to a drug having greater efficacy, treating a broader range of patients or symptoms, or being easier for a patient to use.
Benefits are measured and characterized by clinical trials but may differ when considered from the perspective of each individual patient. Once approved, Lilly may conduct or support additional research to further measure benefits for new uses or in new populations.
What Is Risk?
Pharmaceutical risks are undesirable or harmful events related to a drug therapy. They may be documented or suspected; common or rare; severe or merely irritating; preventable or endured. We characterize the risks associated with medicines along several dimensions to understand their meaning and significance.
To what degree is the drug viewed as the cause of an adverse event?
How severe are these risks?
How likely are these risks?
What is the longevity of the risk? Does risk increase over time? Does risk persist after the medication is halted?
How preventable are risks?
How certain are we about the information used to characterize risks?
Assessing the Benefit-Risk Balance
When the regulatory agency approves a drug, it concludes that the drug's benefits outweigh its risks for the conditions outlined in the product label and that there is a public health benefit from the medication. On its own, this data does not predict whether an individual patient will specifically benefit from a particular medicine.
When prescribing a medication, a physician makes a benefit-risk evaluation based on the drug's potential benefits outweighing the potential risks. Once therapy is initiated, the patient is monitored to evaluate the benefit-risk balance based on how the drug actually performs for the individual patient.
Even as physicians become experienced at identifying individual patients that will more likely benefit from a specific drug therapy, it is still not always possible to predict whether the drug will have the expected therapeutic benefit or whether side effects will occur in an individual patient.
Learning how individual patients react to a medication is essential for the ongoing development of better treatment practices. Providing feedback to Lilly by reporting adverse events is a key part of the ongoing pharmacovigilance (safety monitoring) process. Information on adverse events is collected, monitored and evaluated by Lilly.
Lilly collects, monitors, and evaluates drug safety information in an effort to help health care providers stay current with what is known about the safety of a medication. This is intended to help health care providers make the best possible decision on the use of a particular medication for their patients, and how to best monitor its use once the patient is started on therapy.
Factors Determining Benefit-Risk
What is the expected and actual (individual) benefit for a specific patient?
What is the weight of evidence that supports that a risk exists? (how much uncertainty exists)?
How serious is the risk (including sequelae)?
How often is it that the risk will occur?
Is the risk preventable?
Will it decrease/disappear after a drug is stopped or will the risk increase over time?
Does the risk outweigh the benefit?
Are there any other treatment options (drug or non-drug) where the benefit-risk balance is more favorable or less favorable?
What are the risks of the disease or condition if not treated?